いろいろ heterogeneous mixture definition chemistry example 194618-Heterogeneous mixture definition chemistry example
Examples of the heterogeneous mixture – Most of the mixtures occurring in nature are heterogeneous For example, the soil is a mixture of hundreds of elements and compounds Its composition changes from place to place Some other examples of the heterogeneous mixture are – rocks, a mixture of kerosene and water, rice and pulses, etc EXAMPLES OF MIXTURES ANDDispersed phase and continuous phase The insoluble particles of a colloid do not settle down completely as the particles are small in size, usually ranging between 107 to 103 cm Ans A mixture which has non uniform composition throughout its mass is called heterogeneous mixture The word "hetero" means "different" For example, when you add some mud or sand in the water, you form a heterogeneous mixture

Ways To Separate Mixtures Definition Types Homogeneous Heterogeneous Mixture Eschool
Heterogeneous mixture definition chemistry example
Heterogeneous mixture definition chemistry example- For example, while taking a test, the test will only have content from one class Also, there is a time slot set specifically for chemistry and another for English this being an example of when these components in our "mixture" (English and chemistry) are not uniformly distributed Examples of a heterogeneous mixture Colloid A colloid is an example of a heterogeneous mixture where the components exist in two distinct phases;




Homogeneous Mixture Examples In Nature
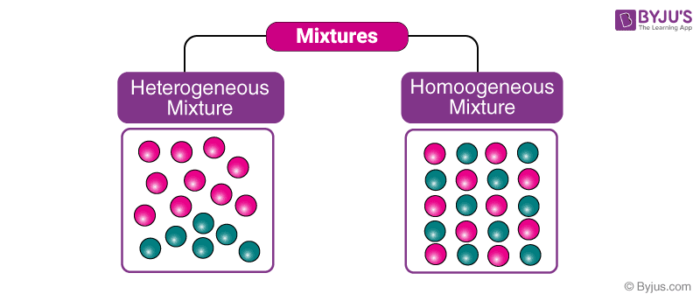
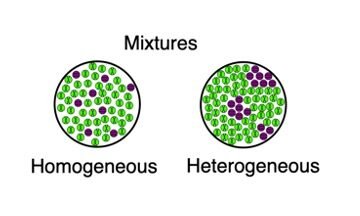
Sea water is a mixture of water, salt and other substances, and air is a mixture of gases such as carbon dioxide, oxygen and nitrogen A heterogeneous mixture is a type of mixture that allows the components to be seen as two or more phases are present A mixture is an example of water Water is a homogeneous mixture of nitrogen, oxygen and smaller amounts of other compounds in the gaseous materialsIn chemistry, we can have two types of mixtures homogeneous mixtures and heterogeneous mixtures Homogeneous mixture Blended so thoroughly, it looks like one substance – Uniform composition Heterogeneous mixture Not thoroughly blended, so you can see and pick out an individual part of the mixture
Homogenous is derived from the Latin term, homogeneus, meaning same or kind In defining what constitutes a mixture, a mixture is formed when two or more compounds or elements are combined without the occurrence of chemical bonding or alterations Used in chemistry, homogenous means having a uniform composition, therefore, a homogenous mixture In chemistry, a mixture refers to the Heterogeneous Mixtures Unlike homogeneous mixtures, in these it is very easy to identify, even to the naked eye, which are the different components that make them up This makes it much easier to separate these mixtures Another example of a heterogeneous mixture is soil, which is composed of various elements and minerals clumped together in seemingly random arrays Here's another example of a heterogeneous mixture – cereal in milk
Bowl of cereal The classic chemistry class example of a multiphase mixture is a bowl of cereal Here you have a heterogeneous mixture of solid cereal in liquid milk carbonated liquid Carbonated water (or carbonated anything) is a multiphase heterogeneous mixture, with gaseous carbon dioxide (CO 2 ) bubbling through liquid water (H 2 O)A chemical mixture combines two substances that maintain their own properties when combined Heterogeneous mixtures are made up of a nonuniform composition, while homogeneous mixtures are made up of a uniform composition For example, water and sand is a heterogeneous mixture — you can easily separate the sand from the water A heterogeneous mixture is a combination of two or more substances, creating a new substance that has nonuniform composition Within the new substance, the concentration of the constituent substances will vary between regions of the substance, so it is possible for one of the chemicals to be more prominent towards the top of a heterogeneous mixture than at the




Homogeneous And Heterogenous Mixture Definition Examples Diagrams




Mixture Science Definition Properties And Examples
In chemistry, the difference between a homogeneous and heterogeneous mixture is a bit more complicated A homogeneous mixture has a uniform composition with all components in a single phase, while a heterogeneous mixture has a nonuniform composition with components in at least two different phasesView chemistpdf from SCIENCE 440 at SMK Khir Johari TUTORIAL DK014 CHAPTER 10 MATTER SEMESTER 1 DK014 UNIT 11 Definition and Classification LEARNING OUTCOMES a) b) DefineMixture, in chemistry, a physical combination of two or more pure substances (ie, elements or compounds) A mixture is distinguished from a compound, which is formed by the chem




Types Of Mixtures Science At Your Doorstep



Heterogeneous And Homogeneous Mixture Differences Videos Examples
Students will take out their chemistry notebooks (for their notes) We will be talking about mixtures today; Homogeneous Mixture Definition, 7 Characteristics, Properties, Example, And Types 2 Homogeneous Mixture or this solution has the properties that, each constituent substance that serves as its formation has an equation, both in the form of taste, color, and comparison In chemistry, for instance, homogenous describes a mixture wherein the substances are distributed evenly At molecular levels, however, they do not have any chemical bonds between them The most common example of a homogenous mixture around us is air Figure 1 The difference between an element and compound as substances in a mixture



Mixture Science Definition Properties And Examples




Homogeneous Mixture Examples In Nature
Homogeneous and Heterogeneous Mixtures Examples, Classification of Matter, Chemistry Homogeneous and Heterogeneous Mixtures Examples, Classification of Matter, Chemistry Watch laterAn alloy is a mixture of elements that has the characteristic of a metal At least one of the elements mixed is a metal One example of an alloy is steel which is made from a mixture of iron and carbon Suspensions (heterogeneous) A suspension is a mixture between a liquid and particles of a solid In this case the particles do not dissolve 02 PROPERTIES Components of a mixture do not combine chemically, therefore they retain their chemical and physical properties Whereas, the compound is a completely new substance with properties entirely different from that of its components For example, a molecule of water (H 2 O) which is a compound has two atoms of hydrogen (H) and one atom of oxygen (O)




What Is A Homogeneous Mixture Definition And Examples
/GettyImages-548326197-58fe30b63df78ca159cb3f67.jpg)



Heterogeneous Definition Science
Heterogeneous Mixture Mixtures that are not uniform all through are called Heterogeneous Mixture Along these lines, a mixture of soil and sand, sulfur and iron filings, oil and water and so on are heterogeneous as they don't have a uniform composition This is on the grounds that in such a case it has two or more distinct phases Properties A heterogeneous mixture is a mixture made of two or more substances that are combined (mixed) together but not dissolved together It is not the same consistency throughout and can be separatedIn chemistry, a mixture is formed when two or more chemical substances are combined together in such a way that neither of them loses their chemical identity Neither the already existing chemical bonds are broken nor new ones are formed A homogenous mixture is a mixture in which the composition is uniform throughout the mixture




Homogeneous Mixture Definition Examples Tutors Com



Homogeneous Heterogeneous Mixture Definition Examples Selftution
Homogenous Mixtures A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample Often it is easy to confuse a homogeneous mixture with a pure substance because they are bothStart studying Homogeneous mixture vs Heterogeneous mixture Learn vocabulary, terms, and more with flashcards, games, and other study toolsSuspensions a heterogeneous mixture in which the solute particles are so large that they settle out when left undisturbed for sometimeThe particles of a suspension are visible to the naked eye When suspension are stirred, the particles seems to be evenly distibuted throughout the mixture eg sandwater, oilwater, dust particles in air etc




Homogeneous Mixture Examples In Nature



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples
For example, you can make a homogeneous solution of sugar and water, but if there are crystals in the solution, it becomes a heterogeneous mixture Many common chemicals are homogeneous mixtures Examples include vodka, vinegar, and dishwashing liquid Many familiar items are heterogeneous mixturesA heterogeneous mixture is a mixture that composes of components that aren't uniform or they have localized regions that all have different properties Despite the term appearing to be highly scientific, there are various common substances that are heterogeneous mixtures In fact, you've probably seen heterogeneous mixtures in your daily life without even realizing itA mixture of sand mixed with salt is an example of a heterogeneous mixture Heterogeneous mixtures possess different properties and compositions in various parts ie the properties are not uniform throughout the mixture Examples of Heterogeneous mixtures – air, oil, and water, etc 2



1




Heterogeneous Mixture Definition Science Trends
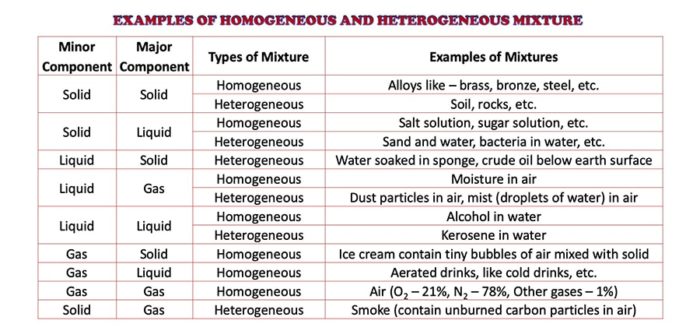
Homogeneous and heterogeneous mixtures In chemistry, if the volume of a homogeneous suspension is divided in half, the same amount of material is suspended in both halves of the substance An example of a homogeneous mixture is air In physical chemistry and materials science this refers to substances and mixtures which are in a single phase A mixture is a substance which consist of two or more elements or compounds not chemically combined together For Example Air is a mixture of gases like oxygen,nitrogen,argon,carbon dioxide etc Gun Powder is a mixture of potassium nitrate,sulphur,charcoal Brass is a mixture of copper and zinc The various substance present in a mixture The scientific definition of a mixture is more complicated than the definition you use in everyday life The degree of combination, the presence or lack of chemical reactions, the size of the particles and the distribution of one substance among another all determine whether something fits the scientific characterization of a mixture and what type of mixture it is
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



10 Heterogeneous And Homogeneous Mixtures




What Is A Heterogeneous Mixture In Science Quora
A heterogeneous mixture can be made into a homogeneous mixture via a process called homogenization An example of homogenization is homogenized milk, which is processed so that the milk components are stable and don't separateTypes of heterogeneous mixture?22 Mixtures (ESAW) We see mixtures all the time in our everyday lives A stew, for example, is a mixture of different foods such as meat and vegetables;




Homogeneous Mixture Examples In Kitchen




10 Examples Of Mixtures
A sample of water that is slushy (partially frozen) is not a homogeneous compound Example 6 Mixtures need not be homogeneous A teaspoonful of sand in water is not a homogeneous mixture Example 7 A homogeneous catalyst is a catalyst that exists in the same phase as the reactants usually liquid phase reactions, sometimes gas phase Example 8 Find heterogeneous mixture stock images in HD and millions of other royaltyfree stock photos, illustrations and vectors in the collection Thousands of new, highquality pictures added every dayOne heterogeneous mixture definition is when substances are not completely mixed all the way down to the molecular level To the naked eye, or perhaps with a microscope, you would be able to see patches of one substance dispersed among patches of another substance Typically, the mixed substances appear as different states, or phases, of matter




Heterogeneous And Homogeneous Mixtures In Cooking And Learning Communities By Natalie King And Brandon Connelly Re Writing Chemistry




Homogenous And Heterogenous Mixtures Chemistry For Non Majors
I will call on people to give me a definition of mixture, homogeneous mixture, heterogeneous mixture, and pure substanceA heterogeneous mixture is a mixture in which the composition is not uniform throughout the mixture Vegetable soup is a heterogeneous mixture Any given spoonful of soup will contain varying amounts of the different vegetables and other components of the soup A phase is any part of a sample that has a uniform composition and properties By definition, a pure substance or a Homogeneous mixture/ Solution A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves as a single substance Another word for a homogeneous mixture is solutionThus, a combination of salt and steel wool is a heterogeneous mixture because it is easy to see which particles of the matter are salt crystals




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures
A heterogeneous mixture Is a mixture which is composed of elements that are nonuniform or have localized regions having different properties In physical chemistry and materials science, the definition of a heterogeneous mixture is that of a mixture Examples of homogeneous mixtures include Salty water — a mixture of salt and water Ruby — a mixture of Al 2 O 3 and Cr 2 O 3 Gasoline — a mixture of various hydrocarbons Brass — a mixture of Cu and Zn Air without clouds — a mixture of various gases Heterogeneous mixtures contain two or more components that can be seen, which canIn this animated lecture, I will teach you about 10 examples of homogeneous mixtures and 10 examples of heterogeneous mixtures, the meaning of homogeneous, t




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Chemistry For Kids Chemical Mixtures
In chemistry, a mixture is a substance that is made up of two or more simpler substances These substances can be chemical elements or compoundsA mixture can be made of liquids, solids, or gases Characteristics A mixture is not the same as a compound which is made of two or more atoms connected together For instance, a mixture of the gases hydrogen and nitrogen contains1 What is a Heterogeneous Mixture?Learn heterogeneous mixture with free interactive flashcards Choose from 290 different sets of heterogeneous mixture flashcards on Quizlet



Mixture




What Are Mixtures Definition Overview Expii
Mixture miks´chur a combination of different drugs or ingredients, as a fluid with other fluids or solids, or of a solid with a liquidA suspension is a heterogeneous mixture of a finely distributed solid in a liquid The solid is not dissolved in the liquid, as is the case with a mixture of salt and water To accelerate the process of sedimentation in a suspension (for example, in chemistry), centrifuges can be used Definition of Heterogeneous Mixtures A mixture is a combination of two or more pure substances in which the original substances retain their chemical properties




Examples Of Homogeneous Mixtures Solid Liquid And Gas




Ways To Separate Mixtures Definition Types Homogeneous Heterogeneous Mixture Eschool



Homogeneous Heterogeneous Mixture Definition Examples Selftution



Inorganic Research Tweet



Heterogeneous Mixture Definition Examples Video Lesson Transcript Study Com




Homogeneous Mixture Definition Lesson For Kids Video Lesson Transcript Study Com




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Sorting Cards




Some Basic Definitions Introductory Chemistry 1st Canadian Edition



What Is A Mixture Definition Types Properties And Examples




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Heterogeneous Mixture Definition Science Trends




Homogeneous Mixture Definition Examples Video Lesson Transcript Study Com
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



10 Heterogeneous And Homogeneous Mixtures




Homogeneous Mixture Examples In Daily Life




Homogeneous Mixture Definition




Homogenous And Heterogenous Mixtures Chemistry For Non Majors



3




Homogenous And Heterogenous Mixtures Chemistry For Non Majors
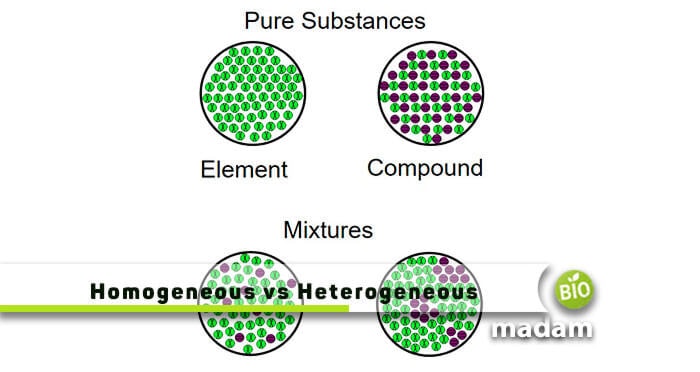



Difference Between Homogeneous Heterogeneous Mixtures Biomadam




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Homogeneous Mixture Examples Found At Home
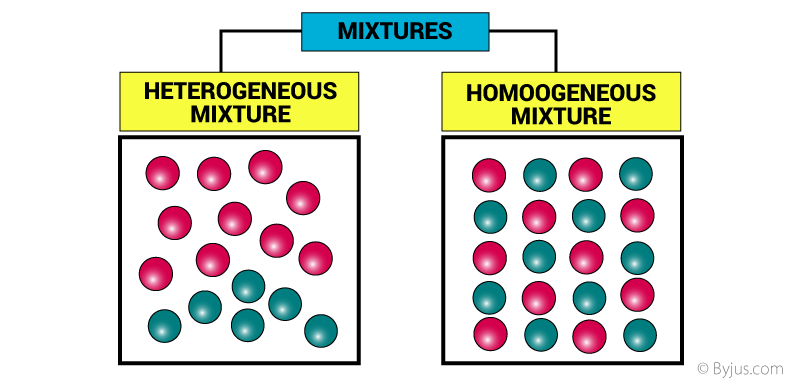



What Is A Mixture Definition Properties Examples Types With Videos




Heterogeneous Mixture Homogeneous Mixture Worksheet Easy Hard Science



1
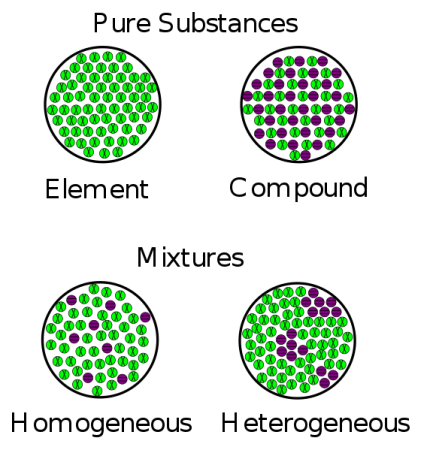



Heterogeneous Mixture Definition Science Trends




Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube
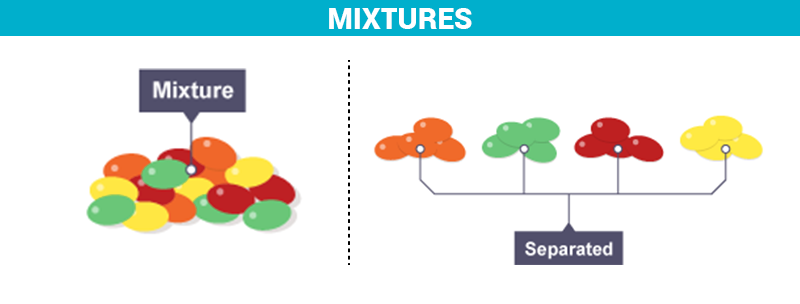



What Is A Mixture Definition Properties Examples Types With Videos




1 In Your Own Words Define The Following Terms Chegg Com




Mixtures And Solutions Cpd Rsc Education




Section 2 Mixtures Of Matter Ppt Download




Homogeneous Mixture Definition Examples Tutors Com




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




Question Video Characteristics Of Heterogeneous Mixtures Nagwa




Heterogeneous Vs Homogeneous Mixtures



Mixture




Topic Mysite




Ways To Separate Mixtures Definition Types Homogeneous Heterogeneous Mixture Eschool




Lesson Explainer Mixtures Nagwa




Heterogeneous Mixture Properties And Examples Udemy Blog




Heterogeneous Mixture And Homogeneous Mixture Youtube
/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples




Heterogeneous And Homogeneous Mixture Differences Videos Examples




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures




Homogeneous Mixture Definition Examples Tutors Com




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Matter Worksheets




Ways To Separate Mixtures Definition Types Homogeneous Heterogeneous Mixture Eschool



Mixture




What Is A Heterogeneous Mixture Definition And Examples




Mixtures Examples Of Mixtures In Chemistry Video Lesson Transcript Study Com




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




Introduction And What Is A Mixture Types Classification Video Examples




Chapter 3 Chemistry



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo
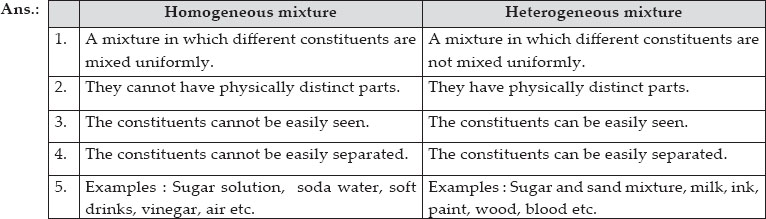



Ncert Solutions Is Matter Around Us Pure Chemistry Class 9



3




Heterogeneous Mixture Lesson For Kids Definition Examples Video Lesson Transcript Study Com
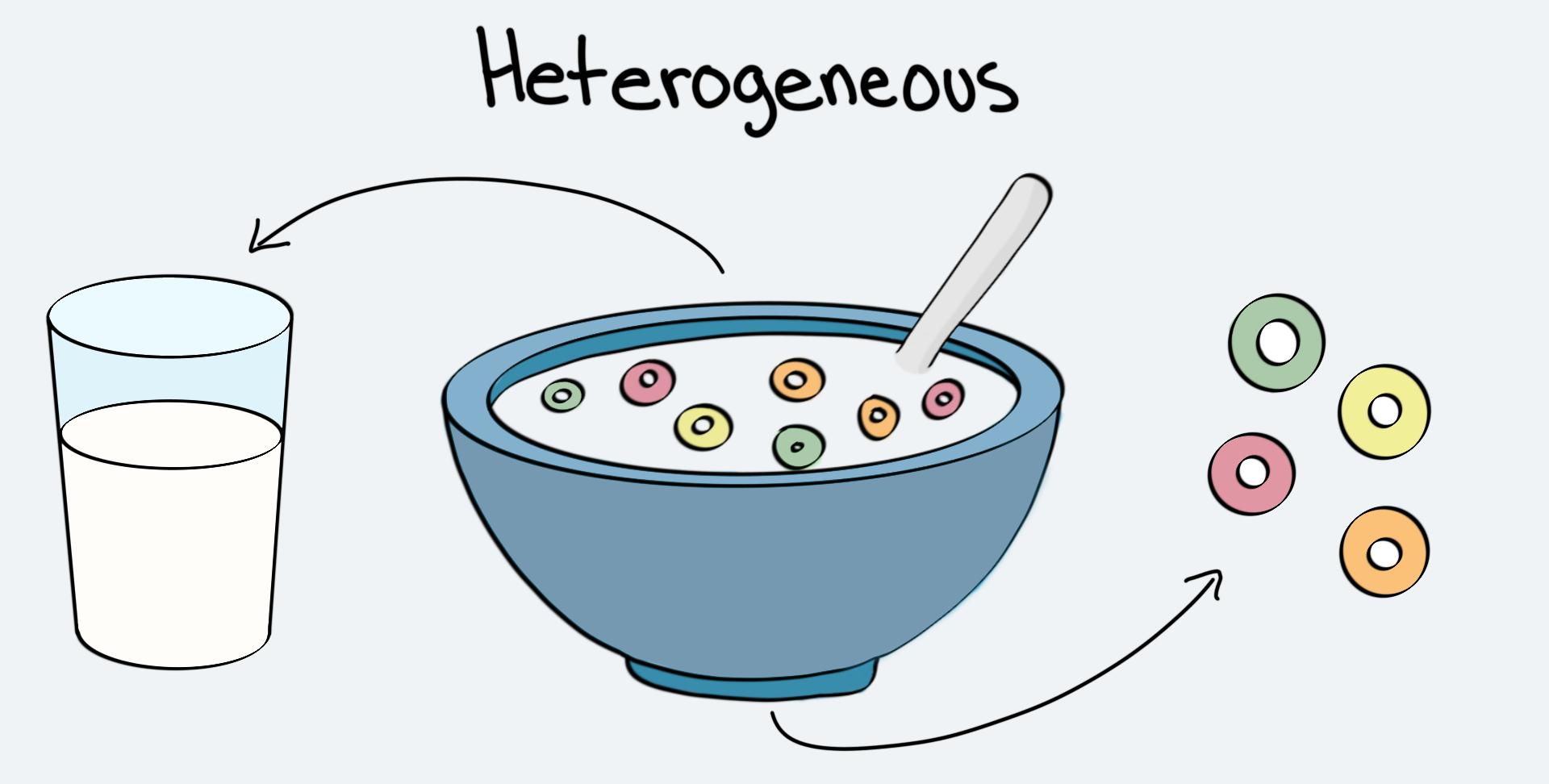



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




5 Examples Of Homogeneous Mixture For Chemistry Class Science Trends




Homogeneous Mixture Examples In Daily Life




Define A Heterogeneous Mixture And Give An Example Chegg Com




Homogeneous And Heterogenous Mixture Definition Examples Diagrams




Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Youtube



Sxxv4y Ycx9hem




Natural And Man Made Examples Of Homogeneous Mixture Science Struck




Is Salty Water Homogeneous Or Heterogeneous




Natural And Man Made Examples Of Homogeneous Mixture Science Struck




Examples Of Heterogeneous Mixtures Types Made Simple




Mixtures And Solutions Cpd Rsc Education




5 Examples Of Heterogeneous Mixtures For Chemistry Class Science Trends




Homogeneous Mixture Definition Examples Tutors Com




3 5 Pure Substances And Mixtures Chemistry Libretexts




5 Examples Of Homogeneous Mixture For Chemistry Class Science Trends




Homogeneous Mixture Definition Examples Tutors Com




Homogeneous Mixture Definition Examples Tutors Com




3 4 Classifying Matter According To Its Composition Chemistry Libretexts



Homogeneous And Heterogeneous Mixture Nine Science
コメント
コメントを投稿